Answer:
(Check explanation)
Step-by-step explanation:
Nickel (II) acetate:
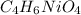
Strontium chlorate:
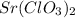
Carbon tetrafluoride:
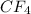
Mercury (II) nitride:
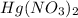
Calcium hydrogen sulfite:
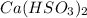
Phosphorus trifluoride:
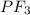
Potassium sulfide:
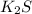
Sodium permanganate:
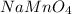
Lithium phosphate:
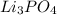
Sulfur dichloride:
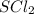
Ionic bonds refer to a bond between a metal and a nonmetal. Therefore, these are ionic:
- Nickel (II) acetate
- Strontium chlorate
- Mercury (II) nitride
- Calcium hydrogen sulfite
- Potassium sulfide
- Sodium permanganate
- Lithium phosphate