Answer: We can make 15.751 grams of HCl.
Step-by-step explanation:
Understanding the Reaction
Before starting a problem like this one, you should always make sure that your equation is balanced. In this case, it was given to us balanced!
PCl₅ + 4H₂O = 5HCl + H₃PO₄
Next, remember that the reactants (on the left) and products (on the right) are equal to each other. So, if you reacted 1 mole of PCl₅ and 4 moles of H₂O, you would produce 5 moles of HCl and 1 mole of H₃PO₄. We can use this information to reason through the rest of the problem.
Limiting Reagent
We are given two amounts of reagents to work with here. It's not very likely that 18g of PCl₅ will be exactly 1 mole and 18g of H₂O will be exactly 4 moles, so we will probably use up all of one before the rest has run out. The reagent that we still have left at the end is called the limiting reagent. We can figure out which reagent will run out first by using proportions.
First, let's convert the grams we were given into moles. We can add up the molecular weights of the elements in each compound to find their molar mass.
PCl₅ = 30.974 + 5*(35.453) = 208.239 g/mol
H₂O = 2*(1.008) + 15.999 = 18.015 g/mol
Next, we'll divide each given weight by the molar mass to find out how many moles we have. Notice that the grams unit will cancel out with dimensional analysis.
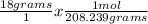 = 0.0864 mol PCl₅
= 0.0864 mol PCl₅
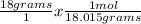 = 0.9991 mol H₂O
= 0.9991 mol H₂O
And lastly, for this step, we're going to compare our newly found amounts of moles with the proportion of moles we need to make our reaction work. To do this, we'll use dimensional analysis again.
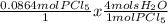 = 0.3456 mols H₂O
= 0.3456 mols H₂O
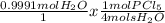 = 0.2497 mols PCl₅
= 0.2497 mols PCl₅
We can interpret these numbers as:
For every 0.0864 mol of PCl₅, we need 0.3456 mol of H₂O and
for every 0.9991 mol of H₂O, we need 0.2497 mol of PCl₅.
Compare the "needed amounts" with the number of moles we're given in the problem. We'll see that if we use up all of the PCl₅, we will still have some H₂O left over. However, if we want to use all of our H₂O (0.9991 mol), we will run out of PCl₅. (0.0864 mol is less than 0.2497 mol)
PCl₅ is our limiting reagent.
Calculating Products from Reagents
Now that we have our limiting reagent, the rest of the problem is simple. We can now use the given amount of PCl₅ to determine how much HCl we can make. Remember that for every 1 mole of PCl₅, we are going to make 5 moles of HCl. We can use that information to set up another dimensional analysis equation.
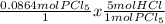 = 0.432 mol HCl
= 0.432 mol HCl
And finally, let's convert back to grams using the molar mass of HCl.
HCl = 1.008 + 35.453 = 36.461 g/mol
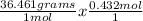 = 15.751 grams of HCl
= 15.751 grams of HCl