Adding 11 g of
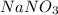 to 25 g of
to 25 g of
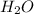 will produce an unsaturated solution at 20 degrees Celsius.
will produce an unsaturated solution at 20 degrees Celsius.
According to the table, the solubility of
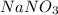 at 20 degrees Celsius is 88 g/100 g
at 20 degrees Celsius is 88 g/100 g
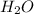 . This means that 88 g of
. This means that 88 g of
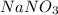 can dissolve in 100 g of
can dissolve in 100 g of
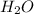 to form a saturated solution at 20 degrees Celsius.
to form a saturated solution at 20 degrees Celsius.
If we add 11 g of NaNO3 to 25 g of H2O, we are effectively adding less than the maximum amount that can be dissolved in 25 g of H2O at 20 degrees Celsius.
To find the maximum amount of NaNO3 that can be dissolved in 25 g of H2O, we can set up a proportion:
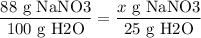
x = 88 x 25/100
= 22 g
Solving for x gives us 22 g of NaNO3.
Since 11 g is less than 22 g, adding 11 g of NaNO3 to 25 g of H2O will produce an unsaturated solution at 20 degrees Celsius.