It's a little hard to determine what the picture says considering it is slightly blurred, however if you are trying to subtract the following, then after simplifying the expression, the solution written in Scientific Notation would be:
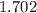 ×
×
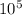
Hope this helps!