Answer:
2264.15 cm³
Step-by-step explanation:
The formula for finding the volume of an object given it's mass and density is given by
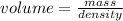
From the question
mass = 1.2 × 10³ grams
density = 0.53 g/cm³
We have
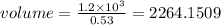
We have the final answer as
2264.15 cm³
Hope this helps you