Answer:
Step-by-step explanation:
The atomic weight of iron (Fe) is 55.845 u. Since it's impossible to count the amount of atoms in a given mass of a substance, we measure atoms in moles. No matter what the element or compound is, there will be
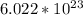 atoms in one mole of compound. This is called Avogadro's number. Atomic weight, also called molar mass, is measured in grams per mole. We first find the number of moles of iron in 3.47 grams by setting up an inequality:
atoms in one mole of compound. This is called Avogadro's number. Atomic weight, also called molar mass, is measured in grams per mole. We first find the number of moles of iron in 3.47 grams by setting up an inequality:
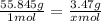 . Solving this inequality shows that there are 0.06213627 moles of iron in 3.47 grams. Now, all we do is multiply that value by Avogadro's number,
. Solving this inequality shows that there are 0.06213627 moles of iron in 3.47 grams. Now, all we do is multiply that value by Avogadro's number,
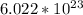 . This gives us 3.7418 * 10^22 atoms in 3.47 grams of iron.
. This gives us 3.7418 * 10^22 atoms in 3.47 grams of iron.