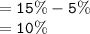Answer:
- Water from beaker will move to the tube
Step-by-step explanation:
Total percentage of water in the beaker;
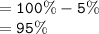
Total percentage of water in dialysis tube;
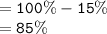
So, there is much water concentration in the beaker than the dialysis tubing, this causes a determined percentage of water to diffuse to the tubing, and determined percentage of salt to move from tubing to the beaker.
Percentage of water moving to tubing;
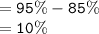
Percentage of salt moving from tubing to beaker