Answer:
No. of moles = 0.0082
Step-by-step explanation:
To calculate the number of moles of a substance given its mass, we can use the following formula:
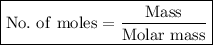
The molar mass of zinc is 65 g/mol. The given mass of Zn in this question is 0.535 g. Using this information and the formula above, we can calculate the number of moles of Zn:
No. of moles =
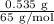
= 0.0082 mol
Therefore, there are 0.0082 mol Zn in 0.535 g of Zn.