Step-1) Identifying the clues given
In this step, let's identify the clues provided in the questions. We have:
- Mass of sample: 90 grams
- Density of sample: 4.5 g/cm³
- To find: The volume of titanium
Since we know the mass and density of titanium, we can apply the density formula to determine the volume of titanium.
Footnote: I have included the formula in case if you need to know it.
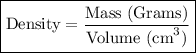
Step-2) Substituting the given clues into the Density formula
To work out the volume of the titanium, we need to substitute the given clues from the question, into the formula. We have:
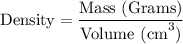
When we substitute the mass and the density of titanium, we have:
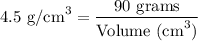
Step-3) Simplify and solve for volume
Finally, let's simplify the equation.
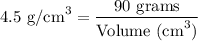
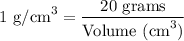
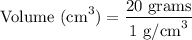
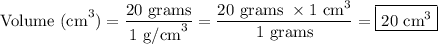
Therefore, the volume of titanium is 20 cm³.