Answer:
1.Usually, density will have units of
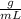
when dealing with a liquid or units of
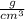 when dealing with a solid.
when dealing with a solid.
The mass has units of grams, g.
The volume will have units of
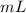 or
or
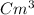
We are given the mass and the volume, both of which have good units, so all we have to do is plug the given values into the equation:
Density =
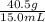
Thus, the block of aluminum has a density of 2.70 g/mL.
------------------------------------------------------------------------------------
2. d = 13.6 g/mL
------------------------------------------------------------------------------------
3. g = (0.789 g/mL) (200.0 mL) = 158 g
------------------------------------------------------------------------------------
4. d = 1896 g / 212.52 cm3 = 8.9 g/cm3
------------------------------------------------------------------------------------
5.d = 1.84 g/mL