Answer:
0.66 L
Step-by-step explanation:
Here it's given that a sample of Neon occupies a volume of 0.220 L at 0.86 atm . And we need to find out its volume at 29.4 kPa .
If we assume temperature to be constant , then we can apply Boyle's Law , which states that ,
" For a fixed amount of gas at constant temperature , pressure is inversely proportional to the volume . "
Mathematically ,
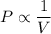
Taking k as constant ,
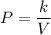
And we can say that ,
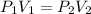
Here ,
- P_1 = 0.87 atm
- P_2 = 29.4 kPa = 29400 Pa
As we know that ,
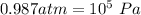
So ,
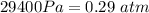
So that ,
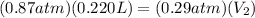
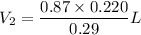
Simplify ,
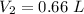
Hence the new Volume of the gas is 0.66 L .
I hope this helps.