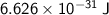So, the amount of energy associated with a photon that has a frequency of 1000 Hz is
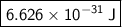
Introduction
Hi ! Here, I will help you to explain the material about photon energy. Photon energy is the energy carried by electromagnetic waves as they propagate. Photon energy is generally affected on frequency (the higher the frequency value, the energy will greater too) or wavelengths (the higher value of the wavelengths, the energy will smaller). So, based on the two concepts above, this is the following equation :
By the Frequency
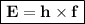
By the Wavelengths
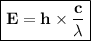
With the following condition :
- E = foton energy (J)
- h = Planck constant ≈
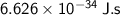
- c = the constant of the speed of light in a vacuum ≈
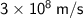 m/s
m/s - f = frequency of electromagnetic wave (Hz)
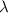 = wavelength (m)
= wavelength (m)
Problem Solving
We know that :
- h = Planck constant ≈
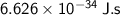
- f = frequency of electromagnetic wave = 1,000 Hz = 10³ Hz.
What was asked :
Step by step :
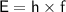
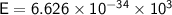
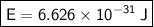
Conclusion
So, the amount of energy associated with a photon that has a frequency of 1000 Hz is