Answer: 2.223 billion years old
Explanation:
since 71 percent of the rock is remaining, the half life hasn't been reached yet. Hence, we know uranium isn't 4.5 billion years old yet. However, every-time 4.5 billions years pass, the rock get smaller by 2 times(a scale factor of 1/2). Hence, you can express that in a log equation:
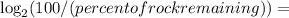 Fraction of 4.5 billions years of time passed
Fraction of 4.5 billions years of time passed
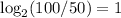 ==> 100% of 4.5 billion years passed. When 50% of rock remains, 4.5 billion years have passed.
==> 100% of 4.5 billion years passed. When 50% of rock remains, 4.5 billion years have passed.
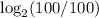 =
=
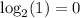 ==> 0% of 4.5 billion years passed. When the rock hasn't decayed at all yet, that means that it is 0 years old.
==> 0% of 4.5 billion years passed. When the rock hasn't decayed at all yet, that means that it is 0 years old.
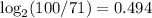 ==> 49.4 % of 4.5 billion years passed
==> 49.4 % of 4.5 billion years passed
0.494*4.5 billion =2.223 billion years passed
2.223 billion years old