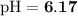Answer:
The pH of the solution is 6.17.
Step-by-step explanation:
To find the pH of the solution, we have to first calculate its pOH using the formula:
![\boxed{\mathrm{pOH = - log_(10)[OH^-]}}](https://img.qammunity.org/2023/formulas/chemistry/college/k3zrri4ul5cnpoq7f81hwih16x5oet7y0q.png)
Substituting the given value of [OH⁻] into the formula, we get:
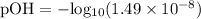
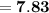
Now that we have the value of the pOH of the solution, we can find its pH using the following formula:
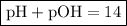
∴
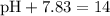
⇒
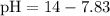
⇒