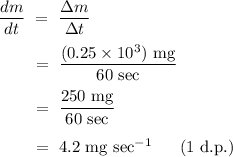
Step-by-step explanation:
The Noyes-Whitney equation is often used to predict the rate at which solid substances, such as drug tablets and granules, in a solvent. Hence, according to the Noyes-Whitney equation, the dissolution rate of a drug tablet or granule can be evaluated in accordance with the following expression
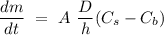 ,
,
where
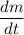 is the rate of dissolution,
is the rate of dissolution,
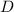 is the dissociation coefficient,
is the dissociation coefficient,
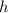 is the thickness of the diffusion layer,
is the thickness of the diffusion layer,
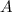 is the surface area of the solute particle,
is the surface area of the solute particle,
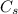 is the particle surface (saturation) concentration and
is the particle surface (saturation) concentration and
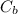 is the concentration in the bulk solvent.
is the concentration in the bulk solvent.
In the case of the question, we need to find the dissolution rate of the drug granules which corresponds to the first-order differential term,
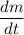 . Looking closely at this term, it implies that the dissolution rate is defined by the change of the solute mass,
. Looking closely at this term, it implies that the dissolution rate is defined by the change of the solute mass,
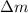 , with respect to the change in time,
, with respect to the change in time,
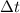 .
.
In accordance with the given description from the question, we know that 0.25g of the 2g drug granules had dissolved into the solution over a period of 1 minute. Using dimensional analysis, we can simply calculate the dissolution rate as