Answer:
58.4 atoms
Step-by-step explanation:
To find the remaining amount of atoms, you need to use the half-life formula:
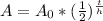
In this formula,
-----> A = remaining amount (atoms)
-----> A₀ = initial amount (atoms)
-----> t = time passed (years)
-----> h = half-life (years)
The half-life is the amount of time that needs to pass for the initial amount of a substance to decay by 50% (or 1/2). According to the graph, 50% of the initial substance is left after 5,730 years.
A = ? atoms t = 16,110 years
A₀ = 410 atoms h = 5,730 years
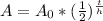 <----- Half-life formula
<----- Half-life formula
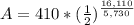 <----- Insert values
<----- Insert values
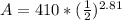 <----- Divide 16,110 by 5,730
<----- Divide 16,110 by 5,730
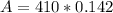 <----- Raise
<----- Raise
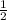 to 2.81
to 2.81
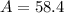 <-----Multiply 410 and 0.142
<-----Multiply 410 and 0.142