Answer:
.90625 moles of Xenon gas
Step-by-step explanation:
The gas is at STP(Standard Temperature & Pressure) of 0 C and 1 atm
When a gas is at STP we know that 1 mol of that gas covers a volume of 22.4L.
Using dimesional analysis we can find the answer:
20.3L Xenon gas ×
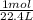 = 0.90625
= 0.90625