Answer: The density is: "0.8 g per cubic centimeter" ;
or: write as "0.8 g per cm³ ."
____
Step-by-step explanation:
Note that:
"
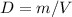 " ;
" ;
that is: Density = mass
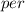 unit volume;
unit volume;
The equation is "mass divided by Volume".
Since even in equation form; the equation is "mass per [unit] volume" ;
We can solve this problem as a "unit rate problem"—albeit within
the conventional standard "units" used as appropriately and customarily.
____
So: Using this equation—Solve for: " ____" :
→
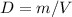
in which:
D refers to: Density ; for which we shall solve.
m refers to: mass ;
[Note: By convention; in units of "grams" [or; "g"] } ;
"V" refers to "Volume";
[Note: By convention; in single "stand-alone units."].
of: "mL" [milliliters] ; or: "cubic centimeters" [cm³ ] ;
→ Note that usually, "mL" is the most commonly used "unit" for measuring liquids/solvents—and that "cm³ "; that is: "cubic centimeters"
—is the most commonly used "unit" for measuring the volume of solids—such as within this very question.}.
____
So, we are given: " m = 24 g "; and: " V = 30 cubic centimeters" ;
____
Plug this into the equation for "Density; "D" ; and solve:
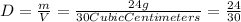
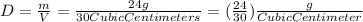 ;
;
↔
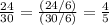 ;
;
Let us convert our fraction to a decimal form; since a density value of a fraction is awkward.
→
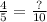 ;
;
To find the "?" value, here is the explanation: We use the value of "10" as the denominator to help convert this value to "decimal form".
Cross-factor multiply.
That is: Given:
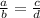 ;
;
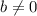 ;
;
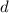
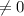 ;
;
⇒ "
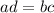 " ;
" ;
Thus: 10(4) = 5(?) ;
→ 40 = 5*(?) ; Now: divide each side by "5" ; to solve for: "?"
40/5 = 5(?) / 5 ;
→ "?" = 8 ;
So: "4/5" = "8/10" ; that is: "(8÷10)" = 8. ÷ 10 = 0.8
The density; D of the object [described in the question]:
is: "0.8 g per cubic centimeter" ;
or: write as: "0.8 g per cm³ " .
____________
Hope this is helpful to you. Best wishes!