Answer :
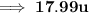
Explanation:
- We know that water is the combination of two hydrogen atoms and one oxygen atoms.
To find atomic mass of water (
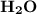 )
)
- Atomic mass of Hydrogen × 2 + Atomic mass of oxygen × 1
We know that,
- Atomic mass of Hydrogen = 1
- Atomic mass of Oxygen = 15.99
 1 × 2 + 15.99 × 1
1 × 2 + 15.99 × 1
 2 + 15.99
2 + 15.99
 17.99u Ans.
17.99u Ans.
Additional information :
Learn the Period table of Elements to solve this type of questions. It is very important. I attached the period table of elements. Learn it and you are able to solve it.