The energy (in MeV) released when decay converts 88x226 into 86y222 is ΔE = 4.87 MeV.
Solution:
Use Energy Conservation. By α decay converts, we mean that the parent particle turns into an alpha particle and daughter particles. Adding the mass of the alpha and daughter radon, we get
m = 4.00260 u + 222.01757 u = 226.02017 u
The parent had a mass of 226.02540 u, so clearly some mass has gone somewhere. The amount of the missing mass is,
Δm = 226.02540 u -- 226.02017 u = 0.00523 u,
which is equivalent to an energy change of,
ΔE = (0.00523 u)
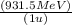 ,
,
ΔE = 4.37 MeV
Hence, the energy released when decay converts is 4.37 MeV.