______________________
| Chemical Reactions - 4.3 |
_______________________
1.) "What are the reactants within a chemical formula?"
- They are the atoms prior to the chemical reaction, found on the left side of the equation.
"Why?"
The substance(s) to the left of the arrow in a chemical equation are called reactants. A reactant is a substance that is present at the start of a chemical reaction. The substance(s) to the right of the arrow are called products. A product is a substance that is present at the end of a chemical reaction
_______________________________________________________
2.) "What does the Law of Conversation of Mass say about a chemical reaction?"
- It states "In a chemical reaction, mass is neither created nor destroyed."
"Why?"
The Law of Conservation of Mass states that in a chemical reaction mass is neither created nor destroyed. For instance, the carbon atom in coal becomes carbon dioxide when it is burned. The carbon atom changes from a solid structure to a gas but its mass does not change.
______________________________________________________
3.) "Which of the following molecules shows two atoms of hydrogen (H)?"
- The molecule
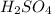 displays two atoms of (H) Hydrogen.
displays two atoms of (H) Hydrogen.
"Why?"
Sulfuric Acid or Sulphuric Acid is a mineral acid consisting of one Sulfur, four Oxygen, and two Hydrogen atoms.
_______________________________________________________
4.) "From the Periodic table, it can be determined that iron (Fe) has an atomic mass of 56 amu, oxygen (O) has an atomic mass of 16 amu, and hydrogen (H) has a mass of 1 amu. What would the atomic mass of Fe(OH)3 be?"
- The atomic mass of Fe(OH)3 would be 107 amu.
______________________________________________________
This should be all correct considering if you are on the same topic as these. However, I hope this helps! If so, lmk! Thanks and good luck!