Answer- 3391.2mile
Explanation:
Greetings !
Firstly, recall distance-speed equation
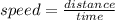
Given values:-
- speed(v)=15.7mile/hr
- time(t)=9days
required values:-
solution /work-out
But, first change the time from day to hour
Thus, 9days=216hours
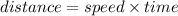
plug in the given values and solve for the distance.
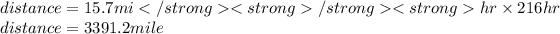
Hope it helps!