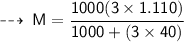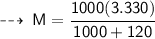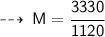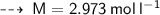
Here we go ~
Question 1
Mass of 1 mole C - 12 atom = 12 g
So, mass of 1 carbon - 12 atom = ( 12 / 1 mole ) g
that is :
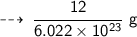
[ since 1 mole = 6.022 × 10²³ ]
Question 2
Molarity :
Molarity is defined as " The number of moles of solute present in per litre of solution "
- Denoted as M = [ moles / litre ]
- change in temperature can cause change in Molarity, as the volume of solution varies with temperature.
- change in pressure can also cause change in Molarity, as volume is affected by pressure as well.
Molality :
Molality is defined as " Number of moles of solute present per kg mass of solvent "
- Denoted as m = [ moles / kg ]
- It isn't affected by any external factors like temperature or pressure, as mass of solvent is constant.
Question 3
As per the given reaction ~
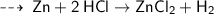
32.65 g of zinc reacted,
[ Number of moles of zinc reacted = mass of zinc reacted divided by its formula Weight ]

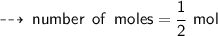
so, we can say that " half mole Zinc reacted with 1 mole of HCl to form half mole of Zinc chloride and half mole of Hydrogen gas "
And we already know that 1 mole of any gas occupies 22.7 litre volume at STP.
So, volume of Hydrogen gas Liberated :
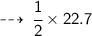
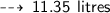
Question 4
The relationship between Molarity and molality can be expressed as :
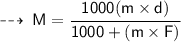
Terms :
- F = formula weight/molar mass = 40 g