240 mL of 15% alcohol solution contains 0.15 • 240 = 36 mL of alcohol.
If we add
 mL of pure alcohol to it, we increase the total volume to
mL of pure alcohol to it, we increase the total volume to
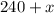 mL, and it will contain
mL, and it will contain
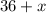 mL of alcohol.
mL of alcohol.
Solve for
 such that the concentration of alcohol (the ratio of the volume of alcohol to total volume of solution) is 80%.
such that the concentration of alcohol (the ratio of the volume of alcohol to total volume of solution) is 80%.
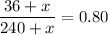
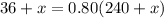
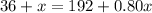
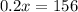
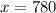
You need to add 780 mL of pure alcohol to get the desired concentration.