Answer:
1.364 M.
Step-by-step explanation:
Molarity formula: M= n/v, where n is moles of solute, and v is liters of solution.
Now, we need to convert the grams of nickel to moles and the volume of water to liters.
125mL/1000= 0.125 L.
To convert nickel grams to moles, we need to take a look at it's chemical formula, which is:
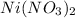
Now we count how many molecules of each element we have:
Ni= 1
N= 2
O= 6
Calculate the weight (g) of each element (the values of g/mol can be found on the periodic table and they may vary slightly between one table and the other):
Ni: (1) (58.6934)= 58.6934
N= (2) (14.007)= 28.014
O= (6) (15.999)= 95.994
Sum all the values to obtain the total weight of 1 mole of this compound:
58.6934+28.014+95.994= 129(g/mole)
Now that we know the that 129 grams equal 1 mole of nickel(II) nitrate, we can convert the 22.0 g to moles:
129g ------- 1 mole
22.0g ----- x
x= (22*1)/129= 0.1705 moles.
Now, we have all the values needed to calculate the molarity of this solution. All we have to do is substitute the values in the formula:
M= (0.1705 moles) / (0.125 L)= 1.364 M.