Answer:
c. 8
Explanation:
let x be the smallest positive integer among the three.
Then
Our three numbers are : x , x + 1 , x + 2
The statement “The multiplication of the three consecutive positive integers equals five times the sum of these numbers”
means
x(x + 1)(x + 2) = 5(x + x+1 + x+2)
⇔ x(x + 1)(x + 2) = 5(3x + 3)
⇔ x(x + 1)(x + 2) = 5×3(x + 1)
⇔ (x + 1)(x + 2)x = 15(x + 1)
⇔ (x + 1)(x² + 2x) = 15(x + 1)
⇔ (x + 1)×[x² + 2x - 15] = 0
⇔ x + 1 = 0 or x² + 2x - 15 = 0 (zero product property used)
Solving x + 1 = 0 :
x + 1 = 0 ⇔ x = -1 (reject because x is positive).
Solving x² + 2x - 15 = 0 (Using quadratic formula) :
Discriminant Δ = 2² - 4(1)(-15) = 64 ⇒ √Δ = 8
Then
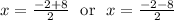
Then
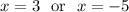
Then
x = 3 (-5 to be rejected because negative)
Conclusion :
The smallest number = x = 3.
The biggest number = x + 2 = 5.
Their sum = 3 + 5 = 8.