Answer:
0.565 nm (3 s.f.)
Step-by-step explanation:
De Broglie Wavelength Formula
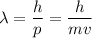
where:
- λ = the de Broglie wavelength (m)
- h = Planck's constant (J s)
- p = momentum of the particle (kg m/s)
- m = mass of the particle (kg)
- v = speed of the particle (m/s)
Planck's Constant
A constant relating the energy of a photon to its frequency:
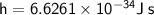
Given:
- v = 7.00 × 10² m/s
- m = 1.675 × 10⁻²⁷ kg
Substitute the given values into the formula (along with Planck's Constant):
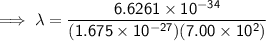
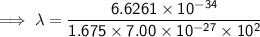
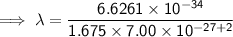
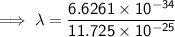
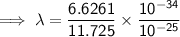
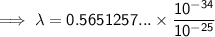
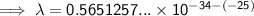
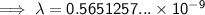
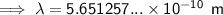
To convert meters (m) to nanometers (nm), multiply by 10⁹:
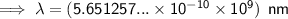
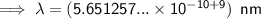
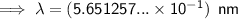
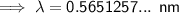
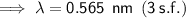
Exponent rules used

