Answer:
6.7 m/s^2
Step-by-step explanation:
The formula of acceleration is:
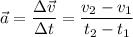
where
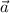 is acceleration,
is acceleration,
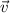 is velocity and
is velocity and
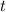 is time.
is time.
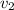 means final velocity.
means final velocity.
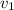 means initial velocity,
means initial velocity,
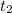 means final time and
means final time and
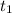 means initial time.
means initial time.
We are given that the Firebird travels at velocity of 0 to 60 mph in four seconds. Therefore:
- Our initial velocity starts at 0 mph.
- Our final velocity is at 60 mph.
- Our initial time is 0 second.
- Our final time is 4 seconds.
Since it travels to the east then our vector will be positive. However, acceleration has to be in m/s^2 unit (Sl unit) so we'll have to convert from mph (miles per hours) to m/s (meters per second) first.
We know that:
- A mile equals to 1609.344 meters.
- An hour equals to 60 minutes which a minute equals to 60 seconds. So 60 minutes will equal to 3600 seconds.
Now we divide 1609.344 by 3600 to find a unit rate of m/s:

Now multiply 0.44704 m/s by 0 and 60 to get velocity in m/s unit:
- Initial velocity = 0 m/s
- Final velocity = 60 * 0.44704 = 26.82 m/s
Time is already in second so no need for conversion. Substitute known information in the formula:
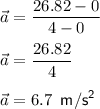
Therefore, the Firebird will accelerate at the rate of 6.7 m/s^2.