Answer:
22.5 g
Explanation:
If there is a 30% concentration of cobalt chloride in 90g of a water and cobalt chloride solution then:
- 30% = cobalt chloride
- 70% = water
Mass of Cobalt Chloride
30% of 90 g
= 0.3 × 90
= 27 g
Mass of Water
70% of 90 g
= 0.7 × 90
= 63 g
If a certain amount of water evaporates (note, the mass of cobalt chloride doesn't change), the ratio of cobalt chloride to water changes to 40% : 60%.
Let x be the new mass the water (in grams).
Therefore:
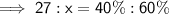
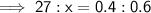
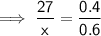
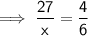
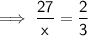
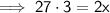
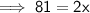
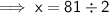
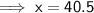
Therefore, the new mass of water is 40.5 g.
To find the mass of water that has evaporated, simply subtract the new mass from the original mass:
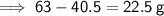
Therefore, the mass, in grams, of water that has to evaporate to have a 40% concentration of cobalt chloride is 22.5 g.