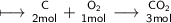#1
Here the basic reaction of C and O_2 occurs hence
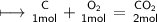
#2
First we have to find moles if Oxygen
- Mass=16g.
- Molar mass=16g/mol
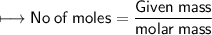
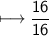
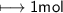
Hence
It will be same like 1st one i. e 2mols of CO_2 will be produced .
#3
Now we already found moles in 16g of dioxygen
Hence