Answer:
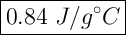
Step-by-step explanation:
Given information
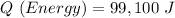
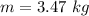
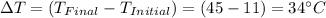
Given formula
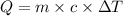
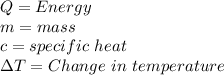
Convert the unit of mass into Grams
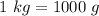
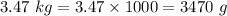
Substitute values into the given formula
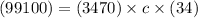
Simplify by multiplication
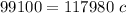
Divide 117980 on both sides
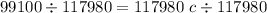

Hope this helps!! :)
Please let me know if you have any questions