Answer:
0.15M
Step-by-step explanation:
The equation for molarity is M= n/L. Where "M" is Molarity, "n" is the number of moles of solute, and "L" is the total liters in solution.
You need to calculate the number of moles from the given grams. The molar mass of KOH is (39.098+ 16 +1.008)= 56.106g. To calculate the mols of KOH,
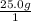 ×
×
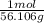 = 0.44558... mol, you see that the grams unit cancel out leaving you with mol as the unit.
= 0.44558... mol, you see that the grams unit cancel out leaving you with mol as the unit.
The volume is given in L already so no need to do any conversion. M=
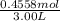 = 0.1485M ≈ 0.15M
= 0.1485M ≈ 0.15M