Answer:
5.5 grams
Step-by-step explanation:
To find how much of the isotope remains, you need to use the half-life equation:
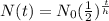
In this equation,
-----> N(t) = remaining mass (g)
-----> N₀ = initial mass (g)
-----> t = time (hrs)
-----> h = half-life (hrs)
You can find the remaining mass by plugging the given values into the equation and solving.
N(t) = ? g t = 30 hrs
N₀ = 22 g h = 15 hrs
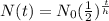 <----- Half-life equation
<----- Half-life equation
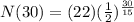 <----- Insert values
<----- Insert values
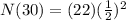 <----- Simplify exponent
<----- Simplify exponent
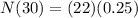 <----- Solve
<----- Solve
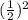
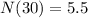 <----- Multiply 22 and 0.25
<----- Multiply 22 and 0.25