Answer:
0.71 moles KI
Step-by-step explanation:
At STP, there should be 1.0 moles at 22.4 L. However, there are only 8.0 L of I₂. Therefore, we must set up a proportion to find the correct moles of I₂.
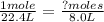 <----- Proportion
<----- Proportion
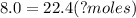 <----- Cross multiply
<----- Cross multiply
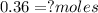 <----- Divide both sides by 22.4
<----- Divide both sides by 22.4
To find the moles of KI, you can use the mole-to-mole ratio from the balanced equation coefficients. The final answer should have 2 sig figs.
2 KI(aq) + Cl₂(g) ---> 2KCl(aq) + 1 I₂(g)
^ ^
0.36 moles I₂ 2 moles KI
---------------------- x -------------------- = 0.71 moles KI
1 mole I₂