Answer:
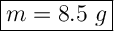
Rounded to the nearest 2 significant figures
Step-by-step explanation:
Given information
Volume of Solution = 417 mL
Molarity of Solution = 0.327 M (mol / L)
Given molecular weight
Mg (Magnesium) = 24 g / mol
F (Flourine) = 19 g / mol
Total Weight (MgF₂) = 24 + 2 × 19 = 62 g /mol
Given formula
m = V × M × n
- m = mass
- V = Volume
- M = Molarity
- n = molar mass (molecular weight)
Convert the unit of volume to Liter
1 L = 1000 mL
417 mL = 417 / 1000 = 0.417 L
Substitute values into the given formula
m = (0.417) × (0.327) × (62)
Simplify by multiplication
m = 136.359 × 62
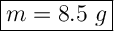
Hope this helps!! :)
Please let me know if you have any questions