Answer: 4010 mol of iron atoms
Step-by-step explanation:
Given Information
Iron Oxide = Fe₂O₃
Mass = 320 kg
Given atomic mass / molecular weight
Fe = 55.8 g / mol
O = 16.0 g / mol
Fe₂O₃ = 55.8 × 2 + 16.0 × 3 = 159.6 g / mol
Given formula
n = m / M
- n = number of moles
- m = mass
- M = molecular weight
Convert the unit of mass to Gram
1 kg = 1000 g
320 kg = 320 × 1000 = 320 000 g
Substitute values into the given formula
n = m / M
n = (320000) / (159.6)
n ≈ 2005 mol of Fe₂O₃
Calculate the number of moles of Fe (Iron atoms)
Since each mole of Fe₂O₃ has 2 moles of Fe, then:
2005 × 2 =
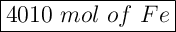
Hope this helps!! :)
Please let me know if you have any questions