Answer:
BeCO3 will hydrolyze in an aqueous medium, yielding a basic solution.
Step-by-step explanation:
In accord with the rules of salt hydrolysis, the cation
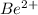 can hydrolyze. The anion
can hydrolyze. The anion
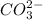 can also hydrolyze, given that the resulting conjugate acid is weak.
can also hydrolyze, given that the resulting conjugate acid is weak.
Given that both ions hydrolyze, the Ka (acid ionization constant) and Kb (Base ionization constant) of each reactant (or product [that is, the conjugate acid and base for each ion]) must be compared.
Using the web (hopefully some sort of reference table has been provided to you):
Ka (for Be^2+) =
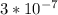
Kb (for CO3^2-) =
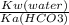
=
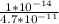
=
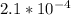
The Kb > Ka, so the solution will be basic.
Hope this helps! My apologies if this answer is incorrect, I have not done this type of problem in a while.