This is an exercise in the general or combined gas law.
To start solving this exercise, we obtain the following data:
Data:
- T₁ = 22.5 °C + 273 = 295.5 K
- P₁ = 1.95 atm
- V₁ = ¿?
- P₂ = 3.69 atm
- T₂ = 11.9 °C + 273 = 284.9 k
- V₂= 56.4 ml
We use the following formula:
P₁V₁T₂ = P₂V₂T₁ ⇒ General formula
Where
- P₁ = Initial pressure
- V₁ = Initial volume
- T₂ = Initial temperature
- P₂ = Final pressure
- V₂ = final volume
- T₁ = Initial temperature
We clear the formula for the initial volume:
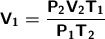
We substitute our data into the formula to solve:
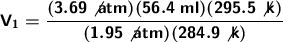
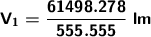

The helium-filled balloon has a volume of 110.697 ml.