Question:
If butane had a volume of 500 ml. at a pressure of 1.5 atm and a temperature of 20 °C, what would the new volume of the gas be at a temperature of 30 °C and a pressure of 500 Torr?
Solution Given:
Let P be the pressure V be volume and and T be temperature.
Volume of Butane [
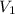 ] = 500 ml
] = 500 ml
Pressure of Butane [
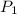 ] = 1.5 atm
] = 1.5 atm
Temperature [
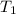 ] =20°C=20+273=293K
] =20°C=20+273=293K
New Volume of Butane [
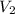 ] = ?
] = ?
New Pressure of Butane [
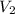 ] =500 Torr=500*0.00131579=0.657895 atm
] =500 Torr=500*0.00131579=0.657895 atm
Note: 1 Torr= 0.00131579 atm
New Temperature of Butane [
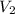 ] =30°C=30+273=303K
] =30°C=30+273=303K
Now
By using combined gas law equation:
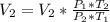
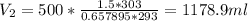
The new volume of Butane is 1178.9 ml