Answer:
15.35 atm
Step-by-step explanation:
Combined Gas Law
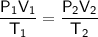
Temperature must be in kelvins (K).
To convert Celsius to kelvins, add 273.15.
Volume can be in any unit.
Given values:
- P₁ = 6.54 atm
- V₁ = 4.5 L
- T₁ = 33 °C = 33 + 273.15 = 306.15 K
- P₂ =
- V₂ = 2.3 L
- T₂ = 94 °C = 94 + 273.15 = 367.15 K
Rearrange the equation to isolate P₂:
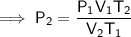
Substitute the given values into the equation:
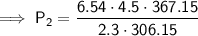
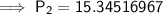
Therefore, the pressure of the gas will be 15.35 atm (2 d.p.).