This is an exercise in the general or combined gas law.
To start solving this exercise, we obtain the data:
Data:
- V₁ = 13 Lt
- T₁ = 25 °C + 273 = 298 k
- V₂ = 27 Lt
- T₂ = 15 °C + 273 = 288 k
- P₁ = 1.3 atm
- P₂ = ¿?
We use the following formula:
- P₁V₁T₂ = P₂V₂T₁ ⇒ General Formula
Where
- P₁ = Initial pressure
- V₁ = Initial volume
- T₂ = Initial temperature
- P₂ = Final pressure
- V₂ = Final volume
- T₁ = Initial temperature
We clear the general formula for the final pressure.
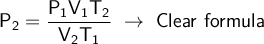
We substitute our data into the formula to solve:
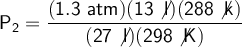
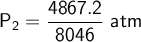
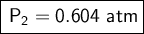
If I measure the pressure after the change by 1.3 atm, the original pressure of the gas will be 0.604 atm.