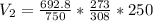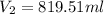Answer:
The volume of the gas sample at standard pressure is 819.5ml
Step-by-step explanation:
Solution Given:
let volume be V and temperature be T and pressure be P.
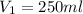
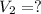
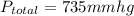
1 torr= 1 mmhg
42.2 torr=42.2 mmhg
so,
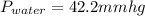
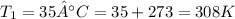
Now
firstly we need to find the pressure due to gas along by subtracting the vapor pressure of water.
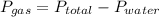
=735-42.2=692.8 mmhg
Now
By using combined gas law equation:
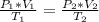
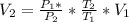
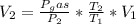
Here
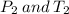 are standard pressure and temperature respectively.
are standard pressure and temperature respectively.
we have
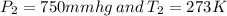
Substituting value, we get