Answer:
0.6258 g
Step-by-step explanation:
To determine the number grams of aluminum in the above reaction;
- determine the number of moles of HCl
- determine the mole ratio,
- use the mole ratio to calculate the number of moles of aluminum.
- use RFM of Aluminum to determine the grams required.
Moles of HCl
35 mL of 2.0 M HCl
2 moles of HCl is contained in 1000 mL
x moles of HCl is contained in 35 mL
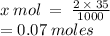
We have 0.07 moles of HCl.
Mole ratio
6HCl(aq) + 2Al(s) --> 2AlCl3(aq) + 3H2(g)
Hence mole ratio = 6 : 2 (HCl : Al
- but moles of HCl is 0.07, therefore the moles of Al;
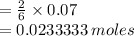
Therefore we have 0.0233333 moles of aluminum.
Grams of Aluminum
We use the formula;
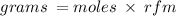
The RFM (Relative formula mass) of aluminum is 26.982g/mol.
Substitute values into the formula;
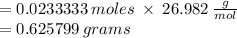
The number of grams of aluminum required to react with HCl is 0.6258 g.