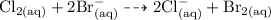Answer:
» Chlorine (Cl) is the oxidizing agent because it gains an electron.
Step-by-step explanation:
Oxidization is the process of losing electrons during an ionic reaction.
In the reaction above, aqueous chlorine captures the electrons of bromide ion hence becoming oxidized. In this sense, since aqueous chlorine is the one gaining electrons, it oxidizes bromide to bromine