Answer:
1.5 moles
Step-by-step explanation:
To find the number of moles of HCl in 500 mL of a 3 M solution of HCl, we consider moles in 1 liter/ 1000 mL.
3 moles HCl is contained in 1000 mL
x moles is HCl is contained in 500 mL
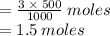
Hence the number of moles of HCl in 500 mL is 1.5 moles.