Answer:

Step-by-step explanation:
Electronic configuration:
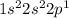
The outermost/valence shell is the second one, seeing from the electronic configuration.
Valence electrons:
= 2 + 1 = 3 (electrons are raised to the power in the electronic configuration)
Since, the valence shell here is 2, the electrons of second shell will be counted as valence electrons.
![\rule[225]{225}{2}](https://img.qammunity.org/2023/formulas/english/college/eq413d752mwtrwwenrzwldxt4w1olmf1b3.png)