Solution:-
- a=Carbon(c)
- b=Nitrogen ion(N+)
- c=Oxygen ion(O-)
Now
#1
Let's look at the EC of carbon(Z=6)
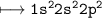
Now.
3 electrons are shared(1 with H_3C and 2 with N+)
Hence
Electron left unshared=4-3=1
#2
Lets look at EC of Nitrogen(N),Z=7
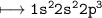
- Valency is 3
- It has already shared 3 electrons
But
Its Cation i.e N+
- Hence 1 electron it donated .Now it has valency 4
Hence
Electron left unshared=4-3=1
#3
Lets look at EC of Oxygen(Z=8)
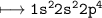
But it is anion hence 1electron required more which us bonded
Hence
Number of electrons left unshared=0