Answer:
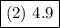
Step-by-step explanation:
Applying the Ideal Gas Equation :
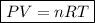
Finding n :
- n = 64 g / 32 g/mole (Oxygen is diatomic)
- n = 2 moles
Solving for P :
- P = 2 × 0.0831 × 300 / 10 (Temp. should be converted to K)
- P = 60 × 0.0831
- P = 4.9
∴ The pressure inside the flask in bar is 4.9.