Answer: See below
Explanation:
To solve for V, we want to first multiply both sides by V, then divide by D.
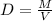 [multiply both sides by V]
[multiply both sides by V]
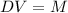 [divide both sides by D]
[divide both sides by D]
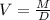
That is the correct answer.
You cannot multiply both sides by M because that would give you M². The whole point is to want to isolate V. In the case of multiplying, you are actually adding more M to the same side. I will demonstrate what it will look like.
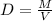 [multiply both sides by M]
[multiply both sides by M]
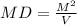
Now, you can multiply both sides by M, and still get the right answer, but that just takes more unnecessary steps. When you say "D=M/V so M/V*M cancels out M snd D*M is Dm=V", that is incorrect. M/V*M DOES NOT cancel out M. It create M² instead.
I hope this answered your question. Let me know if you have anymore questions in the comments.