=> 0.25 M
The question is asking us to find the molarity/concentration of the acid (HCl).
We have been provided with;
12.50 mL of 0.20 M NaOH
10.0 mL of HCl
We know that molarity (M) of a solution is contained in 1 L or 1000 mL or 1000 cm³.
This means that;
0.20 M is contained in 1000 mL.
X mol is contained in 12.50 mL
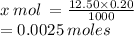
= 0.0025 moles
These 0.0025 moles is contained in 10 mL of HCl.
To find the molarity of the acid;
0.0025 moles is contained in 10 mL
x mol is contained in 1000 mL
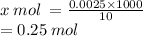
= 0.25 M
Therefore the Concentration of the acid (HCl) is 0.25 M